Discover the basics of nuclear chemistry, including types of radiation, nuclear reactions (fission and fusion), and real-life applications of radioactivity in medicine, agriculture, and power generation. Perfect for students and curious minds!
READ ALSO – Types of Chemical Bonds, Characteristics and Examples
Nuclear Chemistry Explained: Radioactivity, Nuclear Reactions, and Everyday Applications
Nuclear chemistry might sound complicated, but its concepts shape our everyday lives in ways you might not expect. From powering cities to saving lives in hospitals, the principles of radioactivity and nuclear reactions play a crucial role in modern society. In this article, we’ll break down the basics of nuclear chemistry, including the types of radiation, nuclear reactions like fission and fusion, and the various applications of radioactivity in medicine, agriculture, and power generation.
What is Radioactivity?
Radioactivity is the process by which unstable atomic nuclei lose energy by emitting radiation. This happens because certain atoms have too much energy or an imbalance in their neutron-to-proton ratio. To become stable, they release this excess energy in the form of radiation.
Types of Radiation:
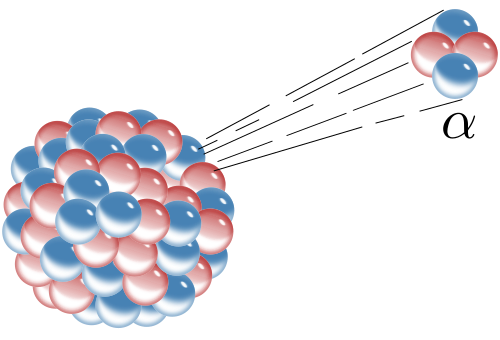
1. Alpha Radiation (α):
- Composed of 2 protons and 2 neutrons (like a helium nucleus).
- It has low penetration power and can be stopped by paper or human skin.
- However, it’s harmful if ingested or inhaled because it can damage internal tissues.
2. Beta Radiation (β):
- Consists of high-energy electrons or positrons.
- It’s more penetrating than alpha radiation but can be stopped by plastic or thin metal sheets.
- Beta particles are used in medical tracers and cancer treatment.
3. Gamma Radiation (γ):
- A form of electromagnetic radiation with high energy.
- It has high penetration power, requiring thick lead or concrete for shielding.
- Gamma rays are used in medical imaging and sterilization.
Half-life Explained
The half-life of a radioactive substance is the time it takes for half of the atoms in a sample to decay. For example, if a radioactive isotope has a half-life of 10 years, only half of the original amount will remain after 10 years. Understanding half-life is crucial in applications like dating archaeological finds and managing nuclear waste.
Nuclear Reactions: Fission and Fusion
Nuclear reactions involve changes in an atom’s nucleus, releasing a tremendous amount of energy. There are two primary types: fission and fusion.
Nuclear Fission
- Definition: Fission is the process of splitting a heavy atomic nucleus into two lighter nuclei, releasing energy.
- How it Works: When a heavy nucleus (e.g., Uranium-235) absorbs a neutron, it becomes unstable and splits, releasing energy and more neutrons. These neutrons can cause further fission, leading to a chain reaction.
Applications
- Nuclear power plants use controlled fission reactions to generate electricity.
- Atomic bombs utilize uncontrolled fission chain reactions for massive explosions.
Nuclear Fusion:
- Definition: Fusion is the joining of two light atomic nuclei to form a heavier nucleus, accompanied by the release of energy.
- How it Works: Fusion occurs under extremely high temperatures and pressures, like in the sun, where hydrogen atoms combine to form helium.
Applications and Challenges:
- Fusion promises a nearly limitless and clean energy source but is difficult to achieve on Earth due to the extreme conditions required.
- Experimental reactors (like ITER) are working towards making fusion a viable power source.
Applications of Radioactivity in Everyday Life
1. Medicine:
- Medical Imaging and Diagnosis: Radioactive isotopes are used in diagnostic techniques such as PET scans and X-rays to detect abnormalities within the body.
- Cancer Treatment: Radiotherapy uses gamma rays to target and destroy cancer cells.
- Sterilization: Gamma radiation sterilizes medical equipment, ensuring they are free from bacteria and viruses.
2. Agriculture:
- Food Preservation: Radiation is used to kill bacteria and pests, prolonging the shelf life of food without affecting its nutritional value.
- Improved Crop Varieties: Radiation induces genetic mutations, leading to the development of high-yield and disease-resistant crops.
- Pest Control: The Sterile Insect Technique involves sterilizing male insects using radiation to control pest populations.
3. Power Generation:
- Nuclear Power Plants: Controlled nuclear fission in reactors produces a significant amount of electricity. This method is efficient and emits low greenhouse gases compared to fossil fuels.
- Space Exploration: Radioisotope thermoelectric generators (RTGs) power spacecraft by converting heat from radioactive decay into electricity.
- Future Prospects: Research in nuclear fusion aims to provide a clean, abundant energy source, potentially revolutionizing global energy supply.
Conclusion: The Power and Potential of Nuclear Chemistry
Nuclear chemistry is not just about complex equations and atomic theory—it’s about real-world applications that power our homes, diagnose illnesses, and even help grow better crops. Understanding radioactivity and nuclear reactions opens up a world of possibilities, from clean energy solutions to advanced medical treatments.
However, with great power comes great responsibility. The use of nuclear technology requires stringent safety measures to minimize risks associated with radiation exposure and nuclear waste. As research continues, the potential benefits of nuclear chemistry could redefine how we live, work, and power the future.
Further Reading and Resources:
- World Nuclear Association: Insights on nuclear power and safety.
- International Atomic Energy Agency (IAEA): Guidelines on the safe use of nuclear technology.
- Nuclear Energy Institute: Latest advancements in nuclear power and fusion research.
Nuclear chemistry is more than just science—it’s a gateway to a sustainable future. Whether you’re a student, educator, or simply curious, understanding its principles can give you a new perspective on the world around you.
READ ALSO – Endothermic and exothermic reactions, meaning and examples
Revision Questions and Answers on Nuclear Chemistry
1. What are the three main types of radiation, and how do they differ in terms of composition, penetration power, and shielding requirements?
Answer: The three main types of radiation are:
- Alpha Radiation (α): Composed of 2 protons and 2 neutrons. It has low penetration power and can be stopped by paper or skin. It is dangerous if ingested or inhaled.
- Beta Radiation (β): Consists of high-energy electrons or positrons. It penetrates more than alpha radiation but can be stopped by plastic or thin metal. It is used in medical tracers and cancer treatment.
- Gamma Radiation (γ): Electromagnetic radiation with high energy and high penetration power. It requires thick lead or concrete for shielding. Gamma rays are used in medical imaging and sterilization.
2. Explain the concept of half-life and provide an example of how it is used in real-life applications.
- Answer: Half-life is the time required for half of the atoms in a radioactive substance to decay. For example, in carbon dating, the half-life of Carbon-14 (about 5,730 years) is used to estimate the age of archaeological artifacts by measuring the remaining Carbon-14 content.
3. Differentiate between nuclear fission and nuclear fusion. Which process is currently used in nuclear power plants, and why?
Answer:
- Nuclear Fission: Involves splitting a heavy atomic nucleus into smaller nuclei, releasing energy. It is used in nuclear power plants because it can be controlled and sustained efficiently.
- Nuclear Fusion: Involves combining two light atomic nuclei to form a heavier nucleus, releasing more energy than fission. However, it requires extremely high temperatures and pressure, making it difficult to achieve on Earth.
4. List and explain three applications of radioactivity in medicine.
Answer:
- Medical Imaging: Radioactive isotopes are used in PET scans and X-rays to detect abnormalities within the body.
- Cancer Treatment: Radiotherapy uses gamma rays to target and destroy cancer cells.
- Sterilization: Gamma radiation is used to sterilize medical equipment, ensuring they are free from bacteria and viruses.
5. How is radioactivity used in agriculture for food preservation and pest control?
Answer:
- Food Preservation: Radiation is used to kill bacteria and pests, extending the shelf life of food without affecting its nutritional value.
- Pest Control: The Sterile Insect Technique sterilizes male insects using radiation, reducing pest populations by preventing reproduction.
- Crop Improvement: Radiation induces genetic mutations, leading to high-yield and disease-resistant crops.
