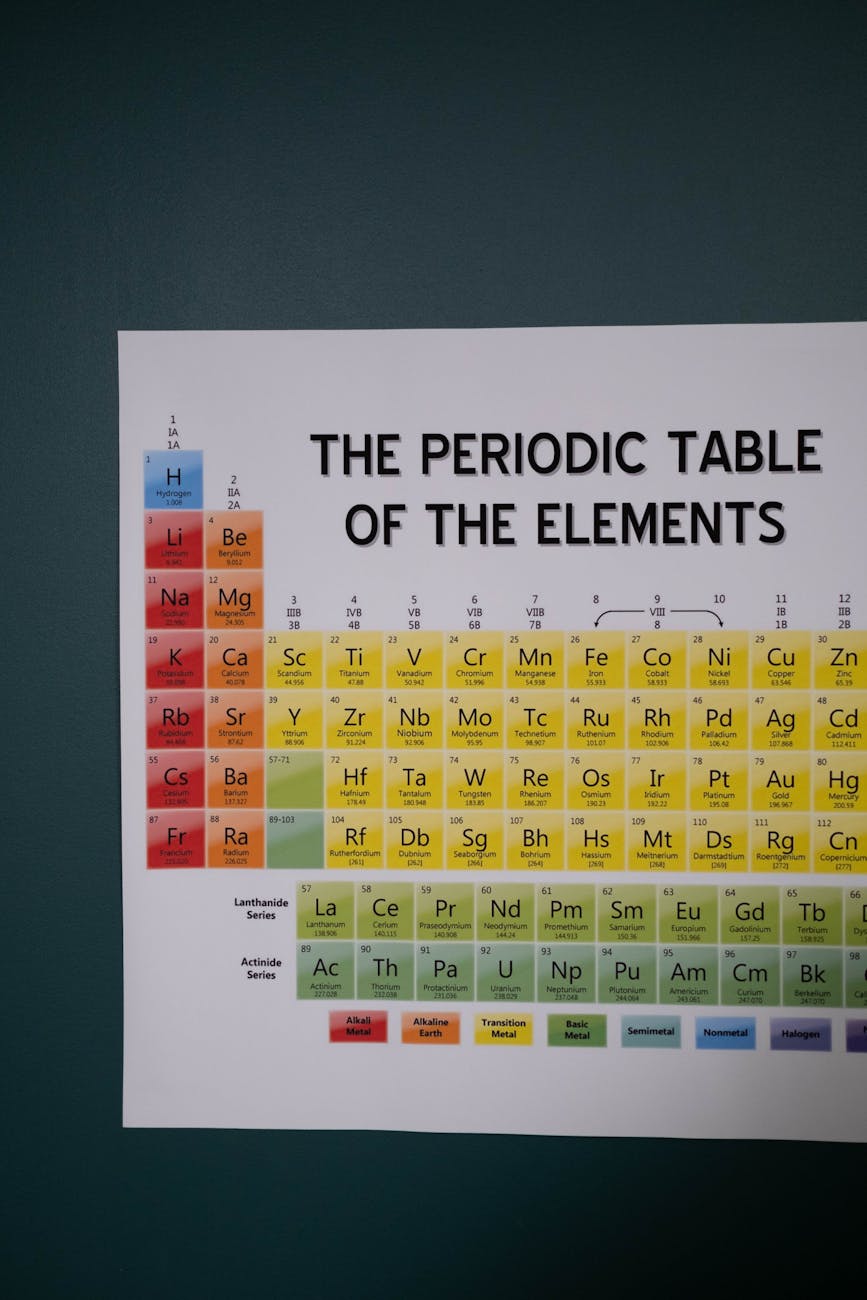
The periodic table is a scientific chart that organizes all known chemical elements based on their atomic number and properties. It is a vital tool in chemistry and science education, used to classify and understand the behavior of elements. At the heart of this arrangement are the 118 chemical elements and their symbols, each representing a unique substance with specific characteristics and applications.
These elements are arranged in periods and groups that reveal trends in atomic structure and chemical reactivity. From metals and nonmetals to metalloids and noble gases, each type of element plays a role in the natural world and in various industries, including healthcare, energy, electronics, and materials science.
Understanding the periodic table of elements and being familiar with element symbols is essential for students, scientists, and anyone interested in how matter is structured. This guide presents all 118 elements along with their symbols to support learning, revision, and a deeper appreciation of the building blocks of matter.
Meaning of Element
An element is a pure substance that is made up of only one type of atom and cannot be broken down into simpler substances by ordinary chemical means. Each element is defined by the number of protons in its nucleus, known as its atomic number, which determines its identity and position in the periodic table.
Elements are the basic building blocks of matter, and everything around us—living or non-living—is composed of one or more elements. For example, oxygen (O) is an element essential for respiration, while iron (Fe) is a key component of blood and steel. Each element has a unique chemical symbol, usually consisting of one or two letters, which is used universally in chemical formulas and equations.
There are 118 known chemical elements, and each one has distinct physical and chemical properties that make it useful in various fields such as medicine, engineering, agriculture, and environmental science.
READ ALSO
Understanding the Periodic Table
The periodic table was first conceptualized by Dmitri Mendeleev in 1869, who arranged elements by atomic mass. Today’s table is organized by atomic number (the number of protons in an atom’s nucleus) and grouped into rows (periods) and columns (groups) based on recurring properties.
Each element is represented by a symbol, usually derived from its English or Latin name. For example, H stands for hydrogen, while Au represents gold (from the Latin aurum).
READ ALSO
118 Elements and Their Symbols: A Complete List
The complete list of all 118 elements and their symbols, arranged in order of their atomic numbers:
| Atomic Number | Element | Symbol | Atomic Mass (approx.) |
|---|---|---|---|
| 1 | Hydrogen | H | 1.008 |
| 2 | Helium | He | 4.0026 |
| 3 | Lithium | Li | 6.94 |
| 4 | Beryllium | Be | 9.0122 |
| 5 | Boron | B | 10.81 |
| 6 | Carbon | C | 12.011 |
| 7 | Nitrogen | N | 14.007 |
| 8 | Oxygen | O | 15.999 |
| 9 | Fluorine | F | 18.998 |
| 10 | Neon | Ne | 20.180 |
| 11 | Sodium | Na | 22.990 |
| 12 | Magnesium | Mg | 24.305 |
| 13 | Aluminum | Al | 26.982 |
| 14 | Silicon | Si | 28.085 |
| 15 | Phosphorus | P | 30.974 |
| 16 | Sulfur | S | 32.06 |
| 17 | Chlorine | Cl | 35.45 |
| 18 | Argon | Ar | 39.948 |
| 19 | Potassium | K | 39.098 |
| 20 | Calcium | Ca | 40.078 |
| 21 | Scandium | Sc | 44.956 |
| 22 | Titanium | Ti | 47.867 |
| 23 | Vanadium | V | 50.942 |
| 24 | Chromium | Cr | 51.996 |
| 25 | Manganese | Mn | 54.938 |
| 26 | Iron | Fe | 55.845 |
| 27 | Cobalt | Co | 58.933 |
| 28 | Nickel | Ni | 58.693 |
| 29 | Copper | Cu | 63.546 |
| 30 | Zinc | Zn | 65.38 |
| 31 | Gallium | Ga | 69.723 |
| 32 | Germanium | Ge | 72.630 |
| 33 | Arsenic | As | 74.922 |
| 34 | Selenium | Se | 78.971 |
| 35 | Bromine | Br | 79.904 |
| 36 | Krypton | Kr | 83.798 |
| 37 | Rubidium | Rb | 85.468 |
| 38 | Strontium | Sr | 87.62 |
| 39 | Yttrium | Y | 88.906 |
| 40 | Zirconium | Zr | 91.224 |
| 41 | Niobium | Nb | 92.906 |
| 42 | Molybdenum | Mo | 95.95 |
| 43 | Technetium | Tc | (98) |
| 44 | Ruthenium | Ru | 101.07 |
| 45 | Rhodium | Rh | 102.91 |
| 46 | Palladium | Pd | 106.42 |
| 47 | Silver | Ag | 107.87 |
| 48 | Cadmium | Cd | 112.41 |
| 49 | Indium | In | 114.82 |
| 50 | Tin | Sn | 118.71 |
| 51 | Antimony | Sb | 121.76 |
| 52 | Tellurium | Te | 127.60 |
| 53 | Iodine | I | 126.90 |
| 54 | Xenon | Xe | 131.29 |
| 55 | Cesium | Cs | 132.91 |
| 56 | Barium | Ba | 137.33 |
| 57 | Lanthanum | La | 138.91 |
| 58 | Cerium | Ce | 140.12 |
| 59 | Praseodymium | Pr | 140.91 |
| 60 | Neodymium | Nd | 144.24 |
| 61 | Promethium | Pm | (145) |
| 62 | Samarium | Sm | 150.36 |
| 63 | Europium | Eu | 151.96 |
| 64 | Gadolinium | Gd | 157.25 |
| 65 | Terbium | Tb | 158.93 |
| 66 | Dysprosium | Dy | 162.50 |
| 67 | Holmium | Ho | 164.93 |
| 68 | Erbium | Er | 167.26 |
| 69 | Thulium | Tm | 168.93 |
| 70 | Ytterbium | Yb | 173.05 |
| 71 | Lutetium | Lu | 174.97 |
| 72 | Hafnium | Hf | 178.49 |
| 73 | Tantalum | Ta | 180.95 |
| 74 | Tungsten | W | 183.84 |
| 75 | Rhenium | Re | 186.21 |
| 76 | Osmium | Os | 190.23 |
| 77 | Iridium | Ir | 192.22 |
| 78 | Platinum | Pt | 195.08 |
| 79 | Gold | Au | 196.97 |
| 80 | Mercury | Hg | 200.59 |
| 81 | Thallium | Tl | 204.38 |
| 82 | Lead | Pb | 207.2 |
| 83 | Bismuth | Bi | 208.98 |
| 84 | Polonium | Po | (209) |
| 85 | Astatine | At | (210) |
| 86 | Radon | Rn | (222) |
| 87 | Francium | Fr | (223) |
| 88 | Radium | Ra | (226) |
| 89 | Actinium | Ac | (227) |
| 90 | Thorium | Th | 232.04 |
| 91 | Protactinium | Pa | 231.04 |
| 92 | Uranium | U | 238.03 |
| 93 | Neptunium | Np | (237) |
| 94 | Plutonium | Pu | (244) |
| 95 | Americium | Am | (243) |
| 96 | Curium | Cm | (247) |
| 97 | Berkelium | Bk | (247) |
| 98 | Californium | Cf | (251) |
| 99 | Einsteinium | Es | (252) |
| 100 | Fermium | Fm | (257) |
| 101 | Mendelevium | Md | (258) |
| 102 | Nobelium | No | (259) |
| 103 | Lawrencium | Lr | (266) |
| 104 | Rutherfordium | Rf | (267) |
| 105 | Dubnium | Db | (270) |
| 106 | Seaborgium | Sg | (271) |
| 107 | Bohrium | Bh | (270) |
| 108 | Hassium | Hs | (277) |
| 109 | Meitnerium | Mt | (278) |
| 110 | Darmstadtium | Ds | (281) |
| 111 | Roentgenium | Rg | (282) |
| 112 | Copernicium | Cn | (285) |
| 113 | Nihonium | Nh | (286) |
| 114 | Flerovium | Fl | (289) |
| 115 | Moscovium | Mc | (290) |
| 116 | Livermorium | Lv | (293) |
| 117 | Tennessine | Ts | (294) |
| 118 | Oganesson | Og | (294) |
The Theory Behind Elements
Each element has a story to tell. For instance:
- Hydrogen (H): The lightest and most abundant element in the universe.
- Gold (Au): Treasured for millennia due to its rarity and beauty.
- Uranium (U): A key player in nuclear energy and weapons.
- Oganesson (Og): The heaviest element, synthesized in laboratories.
Why You should Study the Periodic Table?
The periodic table is more than just a chart; it’s a roadmap to understanding chemistry and the physical world. It helps scientists predict chemical reactions, discover new elements, and develop groundbreaking technologies.
Whether you’re a student, a scientist, or simply curious about the world, the periodic table is a fascinating guide to the elements that make up everything around us.
For students, try to memorize these elements for exam purpose especially and for it to become part of you.
READ ALSO – Chemistry Past Questions: objectives and theories for exam preparation
Conclusion
The periodic table of elements is a fundamental resource in science that organizes the 118 known chemical elements in a way that reveals their structure, properties, and relationships. Each element and its symbol serves as a key to understanding the nature of matter and how substances interact in chemical reactions. From everyday materials to advanced technologies, elements play an essential role in shaping the world around us.
By learning the names and symbols of elements, students and science enthusiasts can build a strong foundation in chemistry and appreciate the importance of elements in life, industry, and innovation. Mastery of this knowledge not only enhances academic performance but also deepens our understanding of the universe at its most basic level.
Revision Questions
- What is the chemical symbol for the element with atomic number 79?
- Identify three elements whose symbols do not start with the same letter as their English names.
- Which group in the periodic table contains elements like He, Ne, and Ar, and what is their common characteristic?
- State the symbols for the first five elements in the periodic table.
- How many elements in the periodic table have one-letter symbols? Give examples.
- Which element has the symbol ‘Fe’ and what is its atomic number?
- Explain the difference between the symbols of Potassium and Phosphorus.
- What is the symbol for the lightest element on the periodic table?
- Which element has the chemical symbol ‘Ag’ and what is its common name?
- Name any two synthetic elements and their symbols.
